‘Embrace the Ditch,’ and Other Lessons Learned in Duke CEE’s Overture Engineering
Civil and environmental engineering students learn to design buildings within less-than-optimal parameters in a collaborative capstone course
We’re sorry, but that page was not found or has been archived. Please check the spelling of the page address or use the site search.
Still can’t find what you’re looking for? Contact our web team »
Read stories of how we’re teaching students to develop resilience, or check out all our recent news.

Civil and environmental engineering students learn to design buildings within less-than-optimal parameters in a collaborative capstone course

On a Star Wars-themed field of play, student teams deployed small robots they had constructed
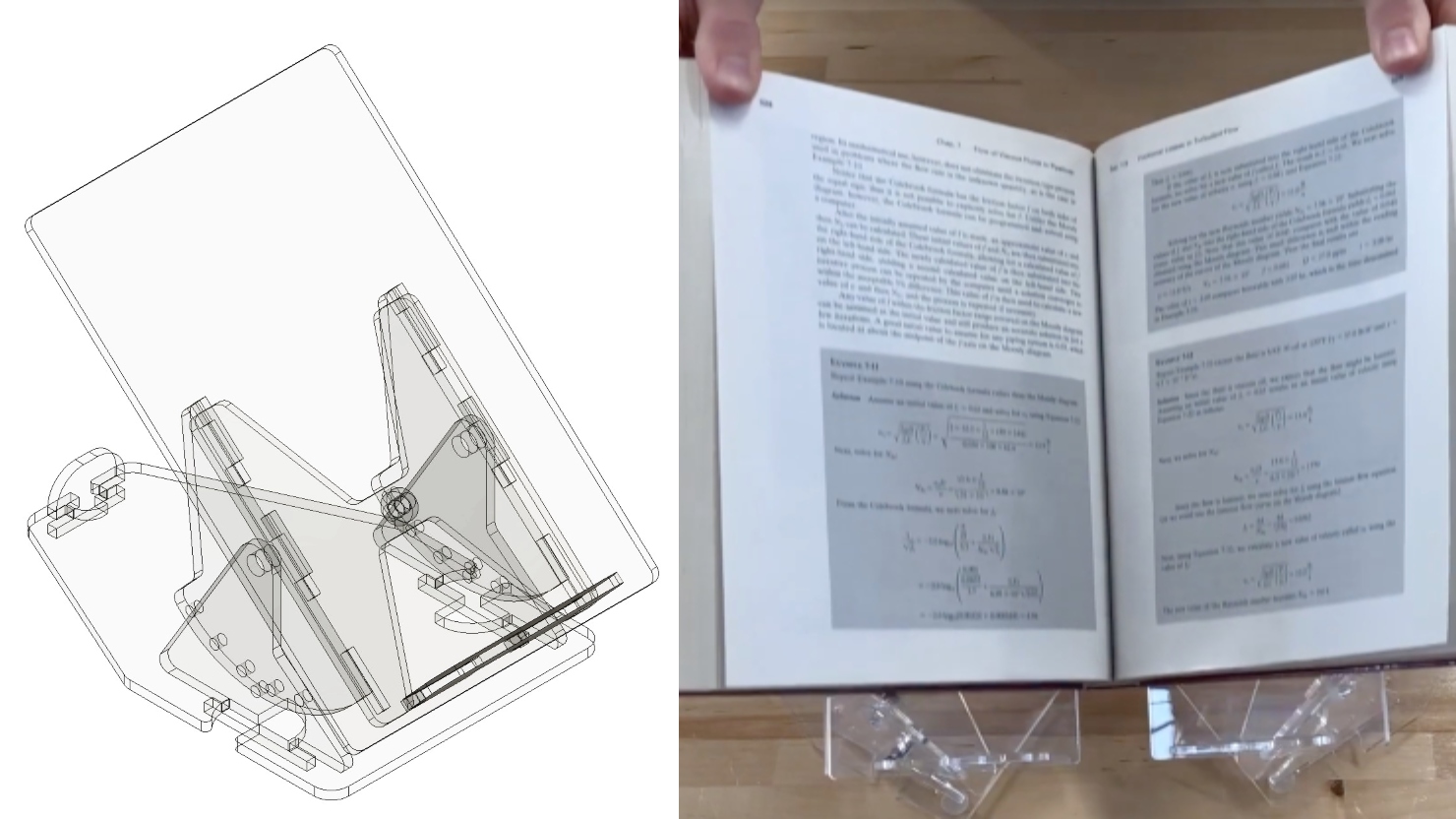
Two projects from First-Year Design course are patent-pending. Student surveys suggest the course also fosters teamwork, leadership and communication skills.
Apr 18
Apr 18
It is estimated that worldwide, there are 100,000 people with cystic fibrosis (pwCF), a genetic-disease which reduces lung function and has historically led to significantly decreased healthspans. Chronic lung infections […]
12:00 pm – 12:00 pm
Apr 18
Summer of 2023 served as a wakeup call for many regarding the impact of extreme heat. The toll on communities was severe. In regions of the US, planes were grounded, […]
12:00 pm – 12:00 pm Virtual